Monday Roundup

From the FEHB Open Season front, consultant Tammy Flanagan reports on the new trend of FEHB plans to offer Medicare Part B premium reimbursement contingent upon joining a related Medicare Advantage plan.
From the Delta variant front, the American Hospital Association informs us that
The Food and Drug Administration Friday authorized another over-the-counter COVID-19 diagnostic test for emergency use. The iHealth COVID-19 Antigen Rapid Test delivers results in 15 minutes. The company anticipates producing 100 million tests per month, with capacity increasing to 200 million per month in early 2022, FDA said.
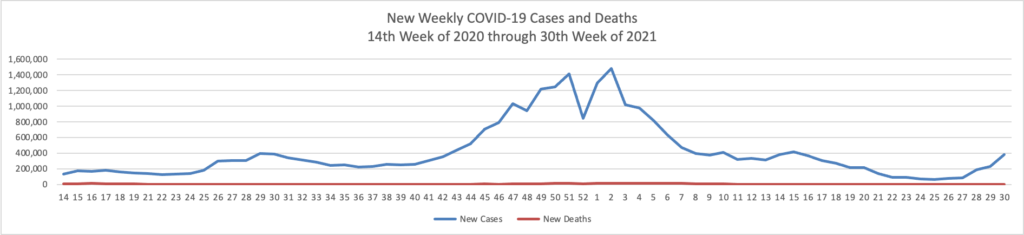
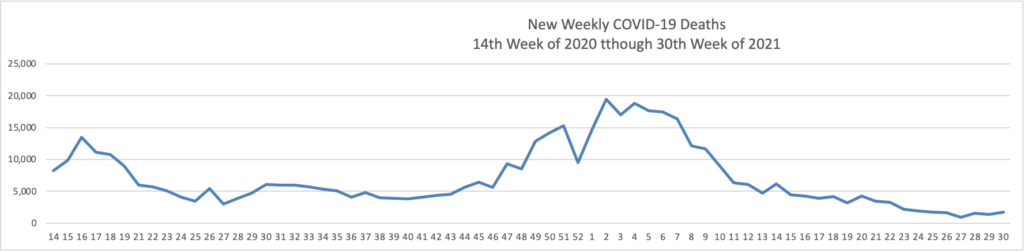
STAT News offers an interesting snapshot of the now diminishing Delta variant surge.

The chart truly speaks for itself.
In the maternal health field, the Health and Human Services Department announced that “200+ hospitals that are participating in the HHS Perinatal Improvement Collaborative, a contract with Premier, Inc. This new network is focused on improving maternal and infant health outcomes by reducing disparities. Comprised of hospitals from all 50 states, the collaborative is the first to evaluate how pregnancy affects overall population health by linking inpatient data of newborns to their mothers.” A list of the participating hospitals may be found at the bottom of the HHS press release.
From the Rx coverage front, STAT News tells us
Nearly a dozen of the highest-rated hospitals in the U.S. charged commercial health insurers and cash-paying patients significantly more than what Medicare has recently paid for 10 infused medicines on which the government spends the most money, according to a new analysis.
Median prices exceeded the Medicare Part B payment limit by a low of 169% at Rush University Medical Center in Chicago, while the Mayo Clinic Hospital in Phoenix exceeded the payment limit by 344%. Among cash-paying customers, the prices ranged from 149% of the Medicare payment limit at Rush to 306% at Brigham and Women’s Hospital and Massachusetts General Hospital, both based in Boston.
The Part B infused medicines for which Medicare Part B spent the most money were Rituxan, Orencia, Enbrel, Prolia, Eylea, Opdivdo, Keytruda, Avastin, Lucentis, Neulasta, and Remicade, but the list did not include biosimilar versions. These medications are variously used to treat conditions including cancer, rheumatoid arthritis, and macular degeneration.
Medicare Part B already sets Part B drug prices which tend to be injectables administered at facilities. Democrat legislators in Congress also want Medicare Part D to fix prices for certain drugs distributed by pharmacies. Government price fixing has never worked successfully in the American economy in the FEHBlog’s understanding.
Also from the healthcare pricing front, Health Payer Intelligence informs
Outcomes-based contracts continue to be popular for certain therapies as healthcare costs mount, an Avalere study found.
Avalere’s findings draw on survey responses from 51 insurers and pharmacy benefit managers. Altogether, the survey participants cover approximately 59 million members. The survey was fielded from September 27 to October 8, 2021 and it is Avalere’s fifth annual survey on the subject.
“OBCs typically include an agreement between health plans and drug or device manufacturers that ties product reimbursement to specific clinical, quality, or utilization outcomes,” Avalere researchers explained.
Let’s go.