Monday Roundup

From the FEHB Open Season front, OPM issued today its annual open season benefits administration letter identifying FEHB and FEDVIP contract changes for 2023 A/K/A, the Significant Changes letter and appendix. OPM also released its Federal Benefits Fast Facts for the upcoming Open Season.
The Federal Times offers an Open Season overview.
From the No Surprises Act front, Newfront, an insurance brokerage, issued an important reminder on the revised NSA consumer notice that health plans must post by January 1, 2023. Here are the current and future notices.
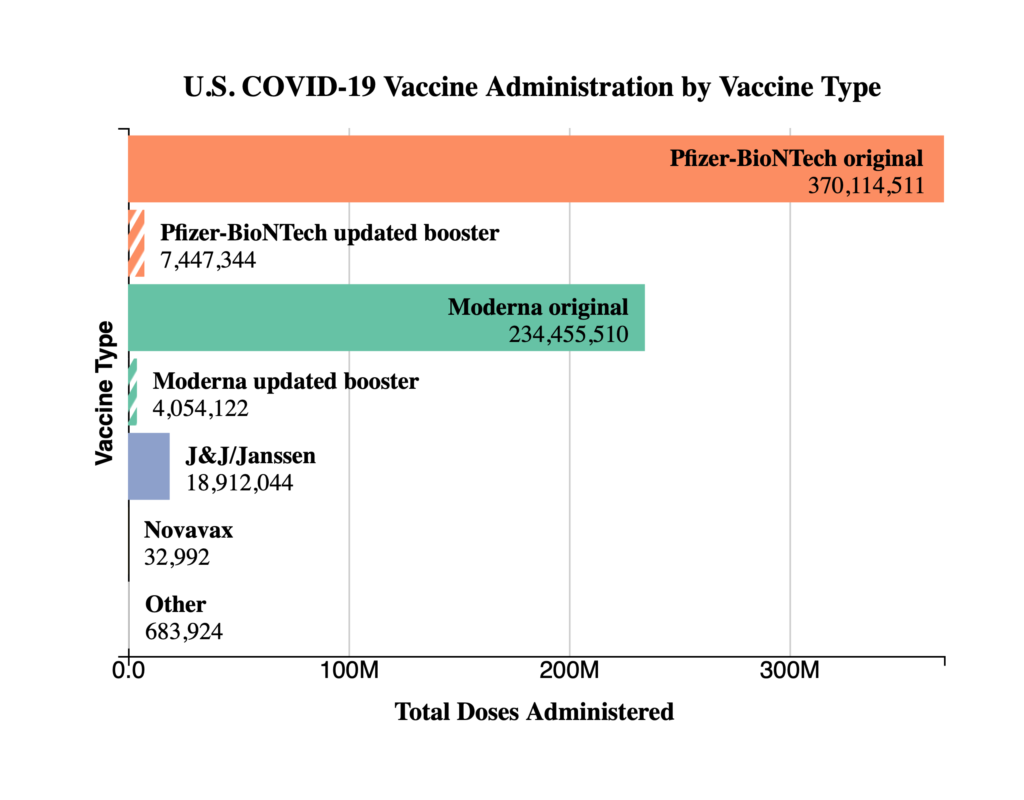
From the Covid vaccine mandate front, the Miller & Chevalier law firm tells us
On October 14, 2022, the Safer Federal Workforce Task Force released a roadmap for federal contractors of anticipated guidance on how federal agencies would be handling the implementation and enforcement of the federal contractor vaccine mandate and workplace safety requirements of Executive Order 14042, “Ensuring Adequate Safety Protocols for Federal Contractors.” The Task Force — created by President Biden to provide guidance to federal agencies on handling operational issues related to the COVID-19 pandemic — anticipates a “potential narrowing of the existing nationwide injunction on October 18, 2022.” As a result, the Task Force anticipates the release of three documents: (1) notice from the Office of Management and Budget (OMB) to federal agencies regarding compliance with injunctions and the inclusion of vaccine mandate clauses in future solicitations and contracts; (2) updates to Task Force guidance on safety protocols for covered contractor and subcontractor workplace locations, including a timeline for implementation; and (3) additional guidance from OMB on “timing and considerations for provision of written notice from agencies to contractors regarding enforcement of contract clauses” implementing vaccine and workplace safety mandates. Notably, until OMB issues the guidance above, agencies are directed not to take any steps to require compliance with the Task Force guidance or enforce any contract clauses implementing the requirements of Executive Order 14042.
This Task Force guidance stems from an August 26, 2022, U.S. Court of Appeals for the 11th Circuit opinion replacing the lower court’s nationwide injunction with an injunction applying to the plaintiffs. However, several other U.S. Courts of Appeals are hearing cases involving this mandate so we may be waiting a while for the OMB guidance.
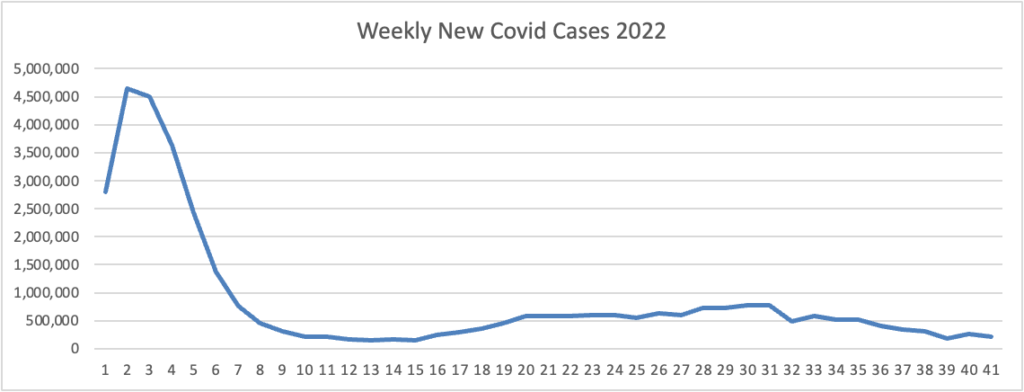
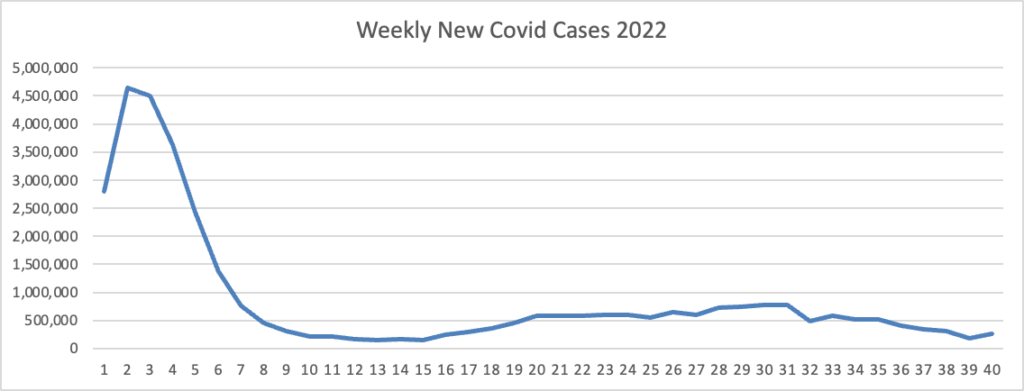
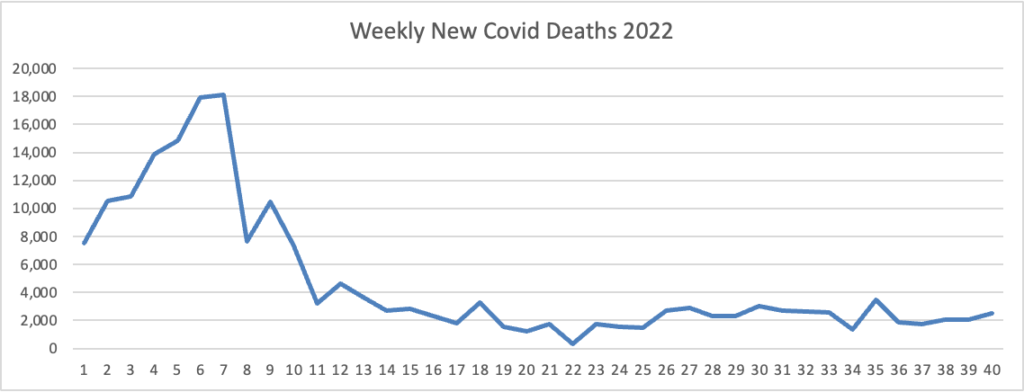
Also, from the Omicron and siblings front, Beckers Hospital Review discusses the new Omicron variants BQ.1 and BQ1.1.
CDC estimates indicate a new omicron variant, BQ.1, and its descendent BQ.1.1 account for 11.4 percent of cases nationwide. The pair have been dubbed “escape variants” for their ability to escape immunity and are currently most prevalent in New York and New Jersey, where they account for nearly 20 percent of new infections. * * *
Experts are optimistic that the bivalent omicron boosters will offer protection against BQ.1 and BQ.1.1 since they’re descendants of BA.5. (Updated boosters are designed to target the original SARS-CoV-2 strain, BA.4 and BA.5.)
“The bad news is that there’s a new variant that’s emerging and that has qualities or characteristics that could evade some of the interventions we have. But, the somewhat encouraging news is that it’s a BA.5 sublineage, so there are almost certainly going to be some cross protection that you can boost up,” Dr. Fauci said.
From the monkeypox front, the American Hospital Association reports
The Centers for Disease Control and Prevention today reported the first U.S. monkeypox case in a health care worker since the outbreak began in May. The report describes how an emergency department nurse in Florida was exposed to the virus through a needlestick, and recommends approaches to preventing infections in health care workers. CDC also released a report describing five patients who acquired ocular monkeypox, a rare but sight-threatening condition, including four who were hospitalized. The report recommends health care providers advise monkeypox patients to practice hand hygiene and avoid touching their eyes, and consider urgent ophthalmologic evaluation and monkeypox-directed treatment for patients with ocular signs and symptoms.
From the influenza front —
Beckers Hospital Review relates
The U.S. is seeing flu activity rise earlier than usual, with Southern states reporting the highest levels of activity, according to the CDC’s latest FluView report for the week ending Oct. 8.
Overall, activity remains low, “but increasing in most of the country,” the CDC said. HHS region 4 (Kentucky, Tennessee, Mississippi, Alabama, Georgia, South Carolina, North Carolina, Florida) and region 6 (New Mexico, Texas, Oklahoma, Arkansas, Louisiana) are reporting the highest levels of flu activity.
Furthermore, STAT News “talked on Friday with Lynnette Brammer, a flu epidemiologist and team lead for domestic surveillance in the CDC’s influenza division, to get a sense of what the agency is seeing.”
Thinking about this flu season and what you’re seeing so far, what’s your best guess for what’s ahead?
Our syndromic surveillance methods are much trickier to try and interpret now, with Covid in the picture. It just muddies the water, basically.
We’ll have to see if the flu and Covid circulate at the same time. Right now, it looks like Covid is still trending down in a lot of the country, but flu’s going up in a lot of the country.
If individuals start to feel crappy this winter, how will they know if it’s a cold? Flu? Covid?
I think testing is going to be really important given that, for flu and Covid, there are treatments that — particularly for high-risk people — can make a huge difference in how well they are able to get through their illness. So it’s going to be really important to test so physicians can know the appropriate treatment for their patients.
In related news, the Government Accountability Office released a report on routine vaccination rates in our country.
U.S. school children generally have higher rates of vaccination to protect them from preventable illness compared with adults.
We found gaps in adult rates for flu, shingles, tetanus, and pneumococcal (prevents pneumonia and more) vaccines. Among other things:
Adults were about 40% more likely to get the tetanus and pneumococcal vaccines than the shingles vaccine
Vaccination rates for Black or African American and Hispanic or Latino adults were about 13% below that of White adults for each vaccine
Health and Human Services is using social media and its website to raise public awareness on the importance of being vaccinated.
From the ACA reporting front, the Internal Revenue Service issued its Forms 1095-B and 1095-C for 2022. The Service also released an employee fringe benefits guide for federal, state, and local government employers.
From the Rx coverage front, BioPharma Dive predicts “five questions facing drugmakers as third-quarter earnings begin. Alzheimer’s study results, drug pricing law, bring new questions for many of the industry’s top companies.”