Monday Roundup

The FEHBlog ran across this Health and Human Services letter to U.S. Governors thanks to the American Hospital Association’s daily email. It states in pertinent part
We are writing to you today to share more details regarding the public health emergency (PHE) for COVID-19, as declared by the Secretary of Health and Human Services (HHS) under section 319 of the Public Health Service Act (42 U.S.C. §247d). The current public health emergency was renewed effective January 21, 2021, and will be in effect for 90 days. To assure you of our commitment to the ongoing response, we have determined that the PHE will likely remain in place for the entirety of 2021, and when a decision is made to terminate the declaration or let it expire, HHS will provide states with 60 days’ notice prior to termination.
All right then.
FCW reports that President Biden “announced a raft of senior officials to help lead the Office of Personnel Management on Jan. 25. The positions are for appointments that don’t require Senate confirmation.” Consequently the lengthy list does not include the OPM Director and Deputy Director nominees. The acting Director remains Chief Management Officer Kathleen McGettigan.
The Washington Post reports that 50-50 Senate Minority Leader Mitch McConnell is ready to approve the “clean” power sharing agreement offered by Senate Majority Leader Chuck Schumer. Why? Two Democratic Senators strongly voiced opposition to repealing the legislative filibuster.
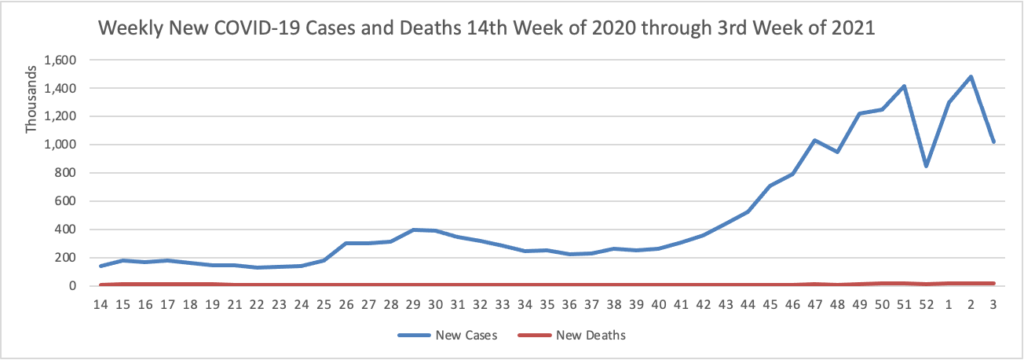
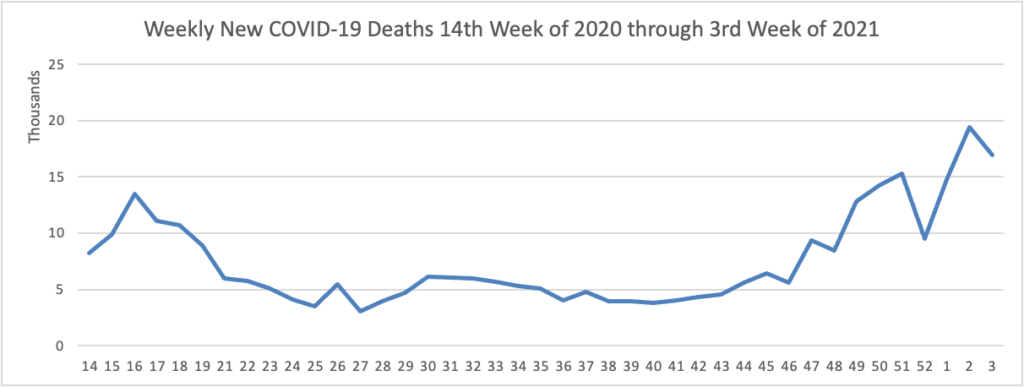
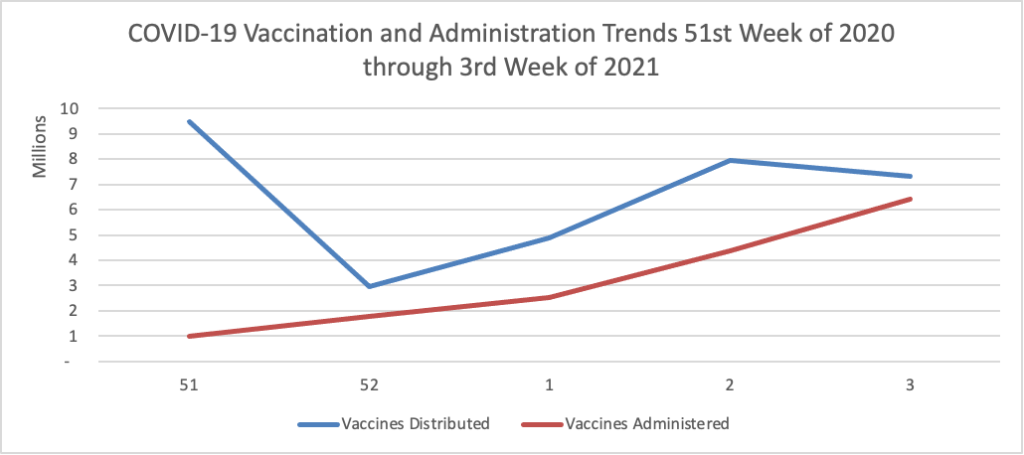
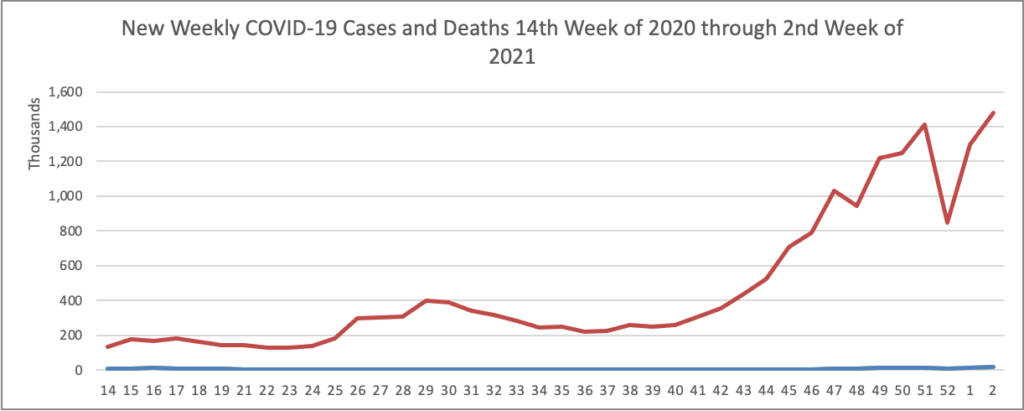
On the COVID-19 vaccination front
- President Biden answering press questions yesterday (more detail at the link and the FEHBlog points this out because for what it’s worth it’s his sense too.)
Q [Josh from Bloomberg] Well, my question was at what date — or, roughly, when do you think anyone who wants [a COVID-19 vaccination] would be able to get it? Summer?
THE PRESIDENT: Oh, I — no, I think it’ll be this spring. I think we’ll be able to do that this spring. And — but it’s going to be a logistical challenge that exceeds anything we’ve ever tried in this country, but I think we can do that.
I feel confident that, by summer, we’re going to be well on our way to heading toward herd immunity and increasing the access for people who aren’t on the first — aren’t on the list, all the way going down to children and how we deal with that. But I feel good about where we’re going, and I think we can get it done.
- Govexec.com reports on efforts by the Department of Veterans Affairs and the Postal Service to arrange for vaccinating their essential workforce members.
- Stat News informs us that “Moderna is studying adding booster doses to its vaccine regimen after finding its Covid-19 vaccine was less potent against a coronavirus variant that was first identified in South Africa, the company said Monday. * * * Nevertheless, [b]oth the Moderna vaccine and the immunization from Pfizer-BioNTech produce such powerful levels of immune protection — generating higher levels of antibodies on average than people who recover from a Covid-19 infection have — that they should be able to withstand some drop in their potency without really losing their ability to guard people from getting sick.”
- NPR News discloses that “Merck is halting development of its two COVID-19 vaccine candidates, saying that while the drugs seemed to be safe, they didn’t generate enough of an immune response to effectively protect people against the coronavirus. * * * While Merck is shelving both of its vaccine candidates, the company says it will keep working on two therapeutic drugs, including one that aims to protect the body’s respiratory system from the coronavirus’s ravaging effects. Last month, the company signed a deal with the U.S. government agreeing to supply up to 100,000 doses of one of those drugs for about $356 million.
In other healthcare news —
- Fierce Healthcare reports that Consulting firm ADVI Health reviewed the websites for 20 (PDF) of the largest hospitals in terms of bed size and found that the largest hospitals all posted some type of pricing information online [in response to a federal transparency rule that took effect on January 1, 2021]. But many of the hospitals did not provide healthcare common procedural codes for the services, according to the analysis, which did not list the hospitals. “Many institutions didn’t use the codes, which makes it difficult to make comparisons across facilities,” said Caitlin Sheetz, lead author of the analysis and head of analytics for ADVI.”
- Fierce Healthcare updates us on that Blue Cross’s new high performance network. “BCBSA said 45 companies have signed on to offer the Blue HPN plan to employees, reaching 55 markets and 340,000 potential members. The plan is the only HPN available in the 10 largest U.S. cities, BCBSA said. Jennifer Atkins, vice president of global network solutions at BCBSA, told Fierce Healthcare that even in its early days, Blue HPN has found success in lowering costs for employers. The HPN plan saved 11% in the total cost of care compared to a traditional PPO, she said.”
- Fierce Healthcare also informs us that non-profit “Civica Rx announced a plan to build a major manufacturing facility to produce sterile injectable drugs for hospitals, a major step for the organization comprised of health systems such as Mayo and Intermountain. The $124.5 million project is planned for Petersburg, Virginia — just south of Richmond — and is expected to potentially create more than 180 jobs. The 120,000-square-foot manufacturing facility aims to address a major source of shortages for hospitals. Sterile injectable drugs have been a major source of shortages in recent years due in part to fewer companies making the products, according to the Food and Drug Administration.”