Weekend Update

Happy Super Sunday LV!
The House of Representatives is engaged in committee work. The Senate is engaged in floor and committee work. The Wall Street Journal reports that
Senate Democrats said they expect a short impeachment trial starting this week for former President Donald Trump as they also seek to push ahead with the Biden administration’s proposed $1.9-trillion economic-relief bill.
The two events, combined with continuing hearings and votes on President Biden’s cabinet nominees, presage competing priorities as the new Democratic majority—which is reliant on Vice President Kamala Harris to break tied votes—is still figuring out key details on the trial process and on what provisions to include in the bill.
The Senate Homeland Security and Governmental Affairs is holding a confirmation hearing for Office and Management and Budget Director nominee Neera Tanden on Tuesday morning. The Senate Health Education Labor and Pension Committee is holding a confirmation hearing for the President’s Education Secretary and Labor Secretary nominees on Thursday morning.
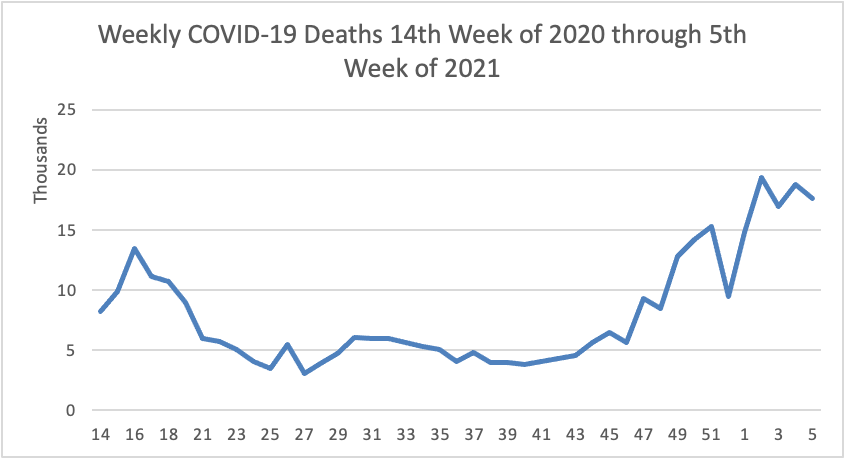
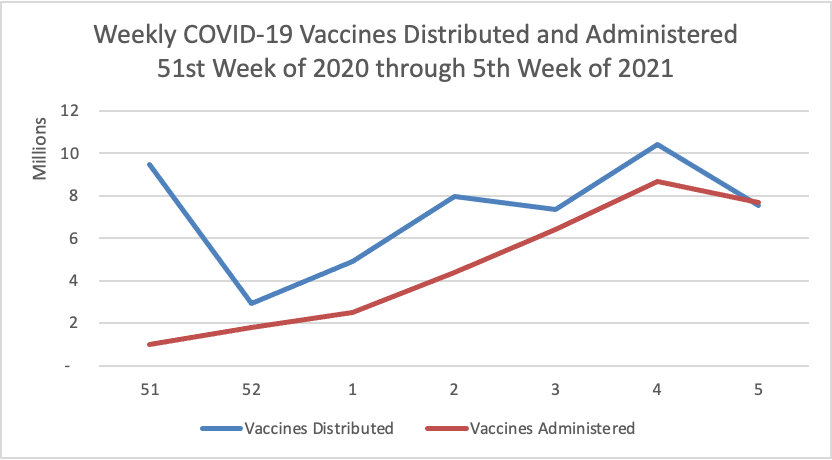
On the COVID-19 front
- The Wall Street Journal’s lead article this afternoon reports that
Vaccination drives hold out the promise of curbing Covid-19, but governments and businesses are increasingly accepting what epidemiologists have long warned: The pathogen will circulate for years, or even decades, leaving society to coexist with Covid-19 much as it does with other endemic diseases like flu, measles, and HIV. The ease with which the coronavirus spreads, the emergence of new strains and poor access to vaccines in large parts of the world mean Covid-19 could shift from a pandemic disease to an endemic one, implying lasting modifications to personal and societal behavior, epidemiologists say.
That’s not a shocker in the FEHBlog’s book. An endemic status would be a vast improvement over what we have faced for the past year.
- Speaking of which, NPR Shots offers the latest advice on upgrading your COVID-19 masks.
“A cloth mask might be 50% effective at blocking viruses and aerosols,” says Linsey Marr, a researcher at Virginia Tech who studies airborne virus transmission. “We’re at the point now … that we need better than 50%.” When you’re outdoors, where fresh air can quickly disperse virus droplets and smaller particles, a cloth mask is still fine, Marr says. But infectious particles can accumulate indoors, and that’s when you want a better mask. “I am now wearing my best mask to the grocery store. I wasn’t before,” Marr says.
The article explains what Ms. Marr means.
- Bloomberg alerts us that
Scientists probing the origins of the coronavirus are wrapping up a lengthy investigation in China and have found “important clues” about a Wuhan seafood market’s role in the outbreak. Peter Daszak, a New York-based zoologist assisting the World Health Organization-sponsored mission, said he anticipates the main findings will be released before his planned [Wednesday] Feb. 10 departure. * * * “It’s the beginning of hopefully a really deep understanding of what happened so we can stop the next one,” he said over Zoom late Friday. “That’s what this is all about — trying to understand why these things emerge so we don’t continually have global economic crashes and horrific mortality while we wait for vaccines. It’s just not a tenable future.”
In miscellaneous healthcare news —
- Investment News informs us that
As every financial professional worth their salt knows, HSAs are a unicorn in the tax world. The accounts are funded with pretax income, grow tax-free and are not taxed when used for eligible expenses — the “triple tax” benefits for which they are so renowned. They represent one of the most efficient ways to squirrel away money for retirement. But they are also kneecapped by their pairing with high-deductible health plans, which are not always optimal for people who regularly anticipate big medical bills.
And while more people have opened HSAs, largely as more employers have opted for high-deductible plans, they are used more like checking accounts than as the long-term saving and investing vehicles they were designed to be.
The pandemic also appears to be having some consequences for HSA use, with those affected by job loss depleting their accounts and those who have remained employed being able to save more than ever, financial advisers say.
The FEHBlog believes that health plans should educate members about getting the most out of their HSA arrangements.
- A friend of the FEHBlog pointed out that on February 5, the Food and Drug Administration “authorized marketing of a new prescription only device [know as eXciteOSA] intended to reduce snoring and mild obstructive sleep apnea. Unlike devices used while patients sleep, this is the first device used while awake that is intended to improve tongue muscle function, which in time can help prevent the tongue from collapsing backwards and obstructing the airway during sleep.”