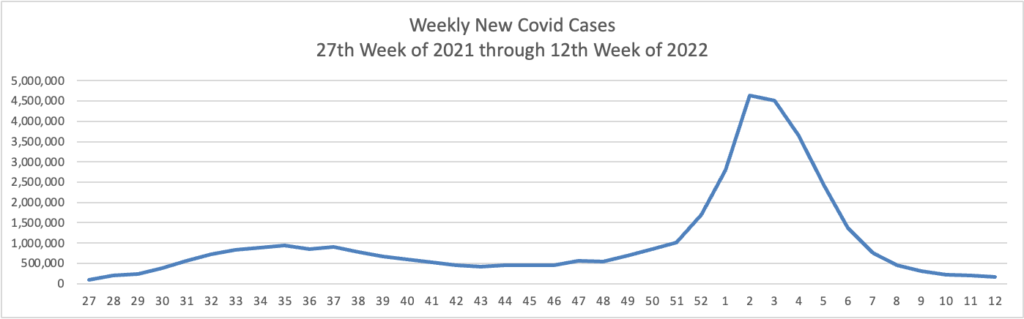
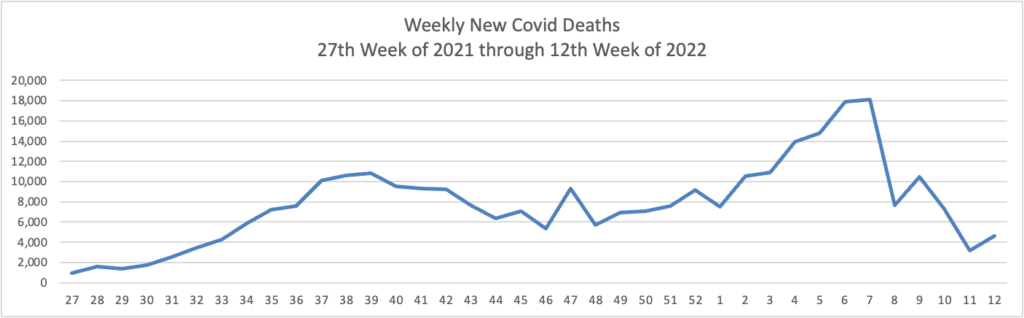
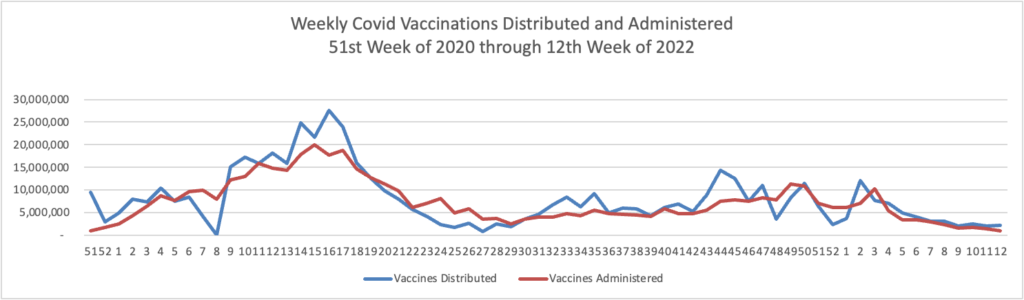
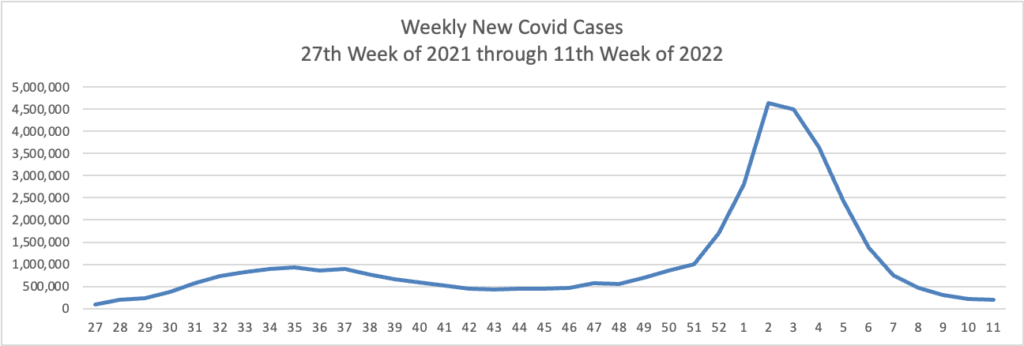
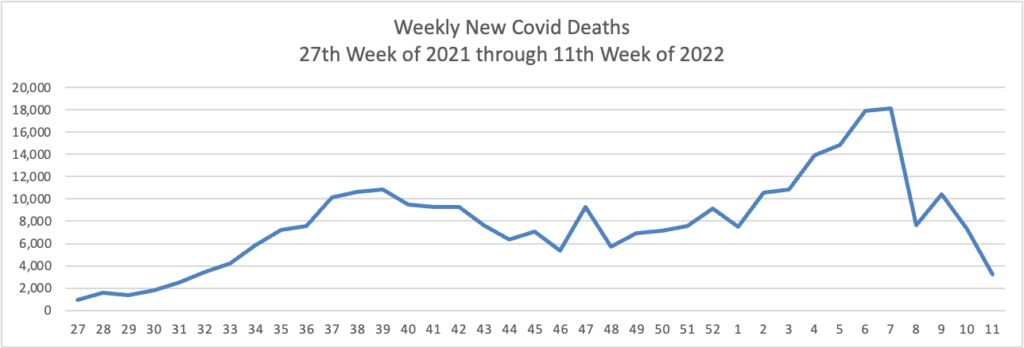
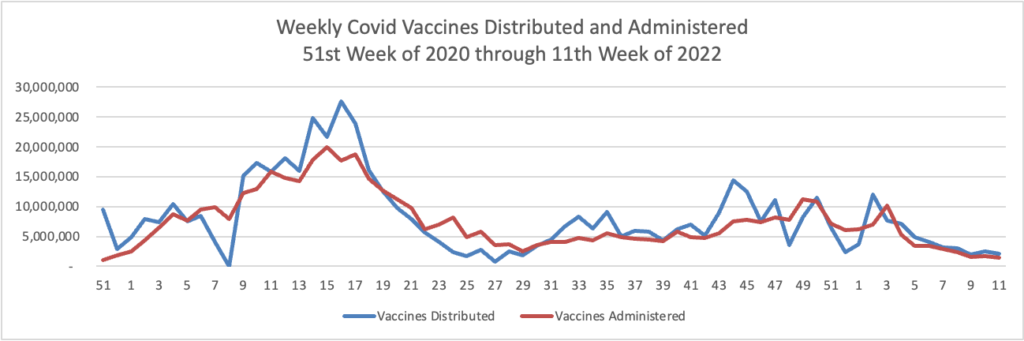
Weekend Update

The House of Representatives and the Senate will be in session for Committee business and floor voting on Capitol Hill this week.
The President will send his fiscal year 2023 federal budget to Capitol Hill tomorrow. The Wall Street Journal offers an explanatory article about this process.
From the federal employment front, Federal News Network discusses the progress of recalling federal employees to their offices.
Also, the FEHBlog noticed that the Federal Times offers a 2022 Federal Benefits Guide — “Answers to commonly asked questions from federal employees, helpful resources, and more.”
From the vaccines front —
- Precision Vaccines informs us “After decades of false starts, new research indicates four Respiratory Syncytial Virus (RSV) vaccine candidates are nearing the completion of late-stage trials. According to the U.S. CDC, RSV vaccines could drastically reduce hospital and intensive-care admissions for young children and seniors.” Fingers crossed.
- NPR Shots tells us
[T]here is now a growing body of research that’s offering at least some reassurance for those who do end up getting infected — being fully vaccinated seems to substantially cut the risk of later developing the persistent symptoms that characterize long COVID.
While many of the findings are still preliminary, the handful of studies that have emerged in the past half year are telling a relatively consistent story.
“It may not eradicate the symptoms of long COVID, but the protective effect seems to be very strong,” says epidemiology professor Michael Edelstein, of Bar-Ilan University in Israel, who’s studying long COVID.
From the mental healthcare front —
- The Wall Street Journal reports “Telemedicine startups make it easier to get ADHD Drugs. That made some [startup] workers anxious. Digital companies such as Cerebral and Done seized on looser pandemic rules for prescribing ADHD drugs like Adderall. Some workers said they felt pressure to provide the medications.”
- Health Payer Intelligence points out
Employee mental healthcare spending rose, and employer mental healthcare spending fell after employers transitioned their workers from preferred provider organizations to high deductible health plans, a study from the Employee Benefit Research Institute (EBRI) found.
The researchers focused on individuals who had been diagnosed with one of three specific mental health conditions to assess the impact of transitioning from a preferred provider organization to a high-deductible health plan: anxiety, attention deficit hyperactive disorder (ADHD), and major depressive disorder (depression).
The study received funding from a handful of organizations, including the Blue Cross Blue Shield Association, the Independent Colleges and Universities Benefits Association (ICUBA), Pfizer, and PhRMA.
[T]he researchers indicated that high deductible health plans could be improved if employers apply value-based care to their high deductible health plan benefit design.
“Smarter deductibles accommodating services preventing the exacerbation of chronic conditions might be a natural evolution of health plans,” the study concluded. “Interventions that improve patient-centered outcomes while maintaining affordability may be found in the form of a clinically nuanced health plan that better meets workers’ clinical and financial needs.”
Because it remains National Kidney Month, the FEHBlog wishes to draw attention to this Fierce Healthcare article about how CVS Health offers personalized kidney care for health plan members.
CVS Kidney Care aims to provide an end-to-end experience to manage kidney care in the long term before it reaches chronic kidney disease or end-stage renal disease. It takes a home-first approach to its care model and is currently co-developing a hemodialysis device that is built specifically with home care in mind.
The device is co-developed by Deka Research & Development and is currently in clinical trials. [CVS Kidney Care President Lisa] Rometty said CVS expects to complete the trial by the end of this year, with anticipated approval and launch sometime in 2023.
CVS chose to get involved in developing the tool, she said, because the company saw an unmet need in the market for a device that’s built from the ground up for in-home dialysis rather than adapted to it. Existing tools are not designed to be easy for a senior patient to understand, for example, Rometty said.
“We did it because we really felt strongly at the time that there wasn’t at the time a device that would meet the unique experience and ease of use and also the safety and clinical aspects,” Rometty said.