Tuesday’s Tidbits

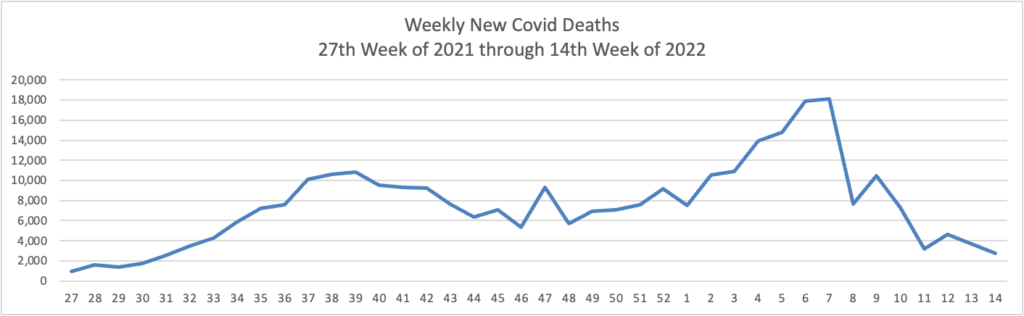
From the Omicron and siblings front —
STAT News tells us
Scientists around the world are discovering and tracking newer forms of the Omicron coronavirus variant, showing how even when a strain becomes globally dominant, it continues to evolve and can splinter into different lineages.
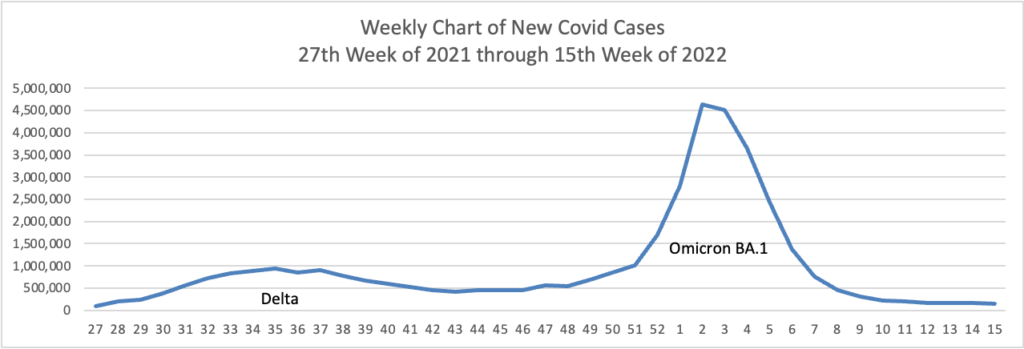
Case in point: Updated data released Tuesday showed that a burgeoning form of Omicron, called BA.2.12.1 — itself a sublineage of the BA.2 branch of Omicron — now accounts for nearly one in five infections in the United States. It’s eating into the prevalence of the ancestral BA.2, highlighting the emergent virus’s transmission advantage over its parent. BA.2 now accounts for about 74% of cases, while the remaining 6% or so are from the BA.1 branch of Omicron, the first form of the variant that took over globally and whose prevalence has been falling as BA.2 became dominant.
The menagerie can be dizzying to track, especially because all these cases technically fall under the Omicron umbrella. But even as scientists closely monitor the divergence of Omicron, early signs suggest the different lineages don’t substantially differ in terms of how virulent they are or in their ability to evade the protection generated by immunizations. While some of the newer forms of the virus might be better spreaders than others, their emergence doesn’t necessarily result in huge increases in cases.
David Leonhardt adds in his New York Times morning column today
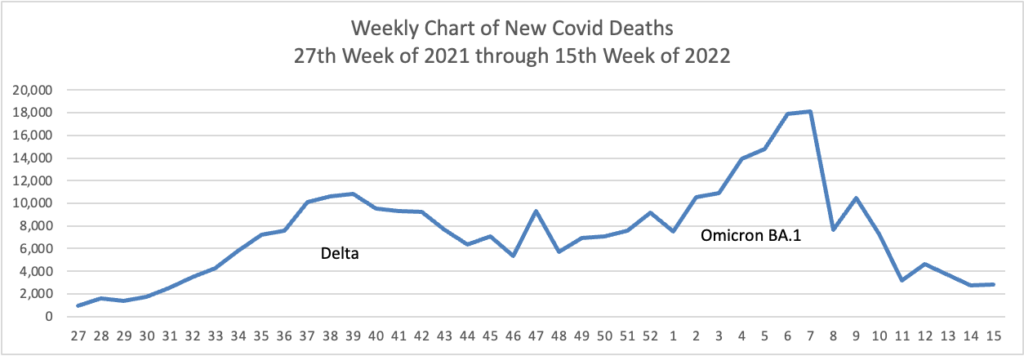
In several places where the number of cases has risen in recent weeks, hospitalizations have stayed flat. (In past Covid waves, by contrast, hospitalizations began rising about a week after cases did.) * * *
Even if hospitalizations do rise in coming weeks, a declining share of coronavirus cases that result in serious illness would be very good news, Dr. Craig Spencer, director of global health in emergency medicine at Columbia University, has pointed out.
I haven’t seen a Covid patient in the E.R. in weeks and go to work now expecting not to,” Spencer told me, “despite a swirl of Covid in the community.”
Among other things, a decoupling of cases and severe illness would mean that hospitals were less likely to become overwhelmed during future Covid surges. When hospitals avoid getting swamped, they can provide care to every patient who needs it — which becomes another factor that reduces bad health outcomes.
For these reasons, Mr. Leonhardt plans to shift his focus from new cases to new hospitalizations.
From the Covid vaccine front
Govexec explains
Because mRNA-based vaccines are a relatively new class of vaccines, they do not include the traditional adjuvants. The current mRNA vaccines used in the U.S. rely on small balls of fat called lipid nanoparticles to deliver the mRNA. These lipid molecules can act as adjuvants, but how precisely these molecules affect the long-term immune response remains to be seen. And whether the current COVID-19 vaccines’ failure to trigger strong long-lived antibody response is related to the adjuvants in the existing formulations remains to be explored.
While the current vaccines are highly effective in preventing severe disease, the next phase of vaccine development will need to focus on how to trigger a long-lived antibody response that would last for at least a year, making it likely that COVID-19 vaccines will become an annual shot.
STAT News adds
New data from Moderna offer hope that booster shots against Covid-19 could become at least somewhat more effective than they already are. But the data also point to how difficult it could be to determine exactly which Covid shots to give as annual boosters.
At a hearing of a Food and Drug Administration advisory panel earlier this month, experts fretted about exactly how governments should make decisions about the composition of annual boosters. And they were adamant that governments, not pharmaceutical companies, should be deciding the strain composition of the shots, as the World Health Organization does for influenza shots. But these data are a reminder that those decisions can be tough. What would experts do when faced with booster shots with several different compositions? Will adding new strains work similarly for different types of vaccines? There are a huge number of open questions.
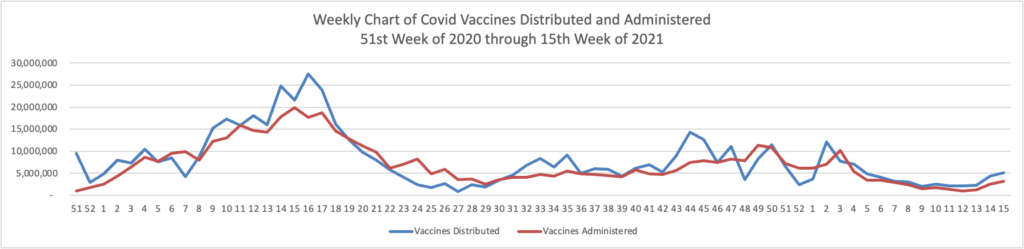
There’s also the biggest problem with annual flu shots: People don’t get them. Even with the current Covid boosters, this has been true. Data presented to the FDA panel said that 217 million Americans are vaccinated about Covid. But only 90 million people have received a booster dose. How many will turn out for a new booster next year?
Look at this comparison of winter 2019-2020 flu vs. 2020-2021 Covid
| 2019 – 2020 Winter CDC Fluview | 3/28/20 | 2020-2021 Winter COVID-19 | 10/1/2020 to 3/21/2021 |
| Flu Deaths | 24,000 | COVID-19 Deaths | 332,636 |
| Flu Cases | 39,000,000 | COVID-19 cases | 22,399,598 |
| Deaths over total cases | 0.06% | 1.49% | |
| https://www.cdc.gov/flu/weekly/index.htm |
Who would look back on pre-Covid flu as the good old days? But comparatively, it is. We see millions more flu cases, but hundreds of thousands fewer flu deaths.
Kaiser Health News discusses the need for better ventilation in office buildings which could help tamp down Covid and flu cases. “The science is airtight,” said Joseph Allen, director of the Healthy Buildings program at Harvard University’s T.H. Chan School of Public Health. “The evidence is overwhelming.”
From the No Surprises Act front, Healthcare Dive reports
The online portal for resolving payment disputes between payers and providers for certain out-of-network charges is now open, the CMS said Monday. The portal initiates what’s known as the federal independent dispute resolution process, a key part of the No Surprises Act that outlaws balance bills in most cases. As a last resort, it allows payers and providers to resolve payment disputes using an arbitration style similar to the model adopted by Major League Baseball in salary negotiations.
From the transparency in coverage rule front, the Labor Department issued ACA FAQ 53 today. FAQ 53 provides guidance to health plans, including FEHB plans, on how to post three machine-readable pricing files on their website. The Labor Department will begin to enforce this requirement on July 1, 2022.
From the healthcare pricing front
Health Affairs reports
Commercial health plans pay higher prices than public payers for hospital care, which accounts for more than 5 percent of US gross domestic product. Crafting effective policy responses requires monitoring trends and identifying sources of variation. Relying on data from the Healthcare Provider Cost Reporting Information System, we describe how commercial hospital payment rates changed relative to Medicare rates during 2012–19 and how trends differed by hospital referral region (HRR). We found that average commercial-to-Medicare price ratios were relatively stable, but trends varied substantially across HRRs. Among HRRs with high price ratios in 2012, ratios increased by 38 percentage points in regions in the top quartile of growth and decreased by 38 percentage points in regions in the bottom quartile. Our findings suggest that restraining the growth rate of HRR commercial hospital price ratios to the national average during our sample period would have reduced aggregate spending by $39 billion in 2019.
Fierce Healthcare relates
Seniors save nearly $2,000 on average a year in total healthcare spending in Medicare Advantage (MA) compared to fee-for-service Medicare, a new study finds.
The study, published Tuesday, by the advocacy group Better Medicare Alliance finds that seniors spent $1,965 less including premiums and out-of-pocket costs on MA when compared to fee-for-service.
“We see particularly strong results for historically disadvantaged populations, including Black and Hispanic beneficiaries and those who are low-income,” said Allison Rizer, principal at the consulting firm ATI Advisory, which performed the study that examined 2019 Medicare Current Beneficiary Survey data.
From the healthcare business front, Fierce Healthcare tells us
UnitedHealth Group executives said Thursday that its Optum Health subsidiary, which is one of the country’s largest physician groups, is building out value-based care partnerships at a faster rate than was expected.
In its earnings report, the healthcare giant said it initially projected that 500,000 new patients would be treated in value-based arrangements. It’s upping that projection to 600,000. Wyatt Decker, M.D., CEO of Optum Health, said on the company’s earnings call that reflects Optum’s efforts to invest in technology, analytics and building networks are paying off.
“What you’re really seeing is a result of almost 10 years of building a flywheel that now has significant momentum,” Decker said. “All of that continues to yield benefits and, frankly, growth.”
From the research front —
MedPage Today announced
The severity of multiple sclerosis (MS) was linked with geographic latitude, an observational study showed.
Among 46,000 MS patients living in temperate zones, more severe disease was seen in those who lived above 40° latitude, reported Tomas Kalincik, MD, PhD, of the University of Melbourne, Australia, and co-authors.
The association was driven mainly, but not exclusively, by ultraviolet B (UVB) radiation exposure contributing to both MS susceptibility and severity, the researchers wrote in Neurology.
AHRQ discusses a study on “Geographic Variation in Inpatient Stays for Five Leading Substance Use Disorders, 2016-2018.” There are interesting State variations.