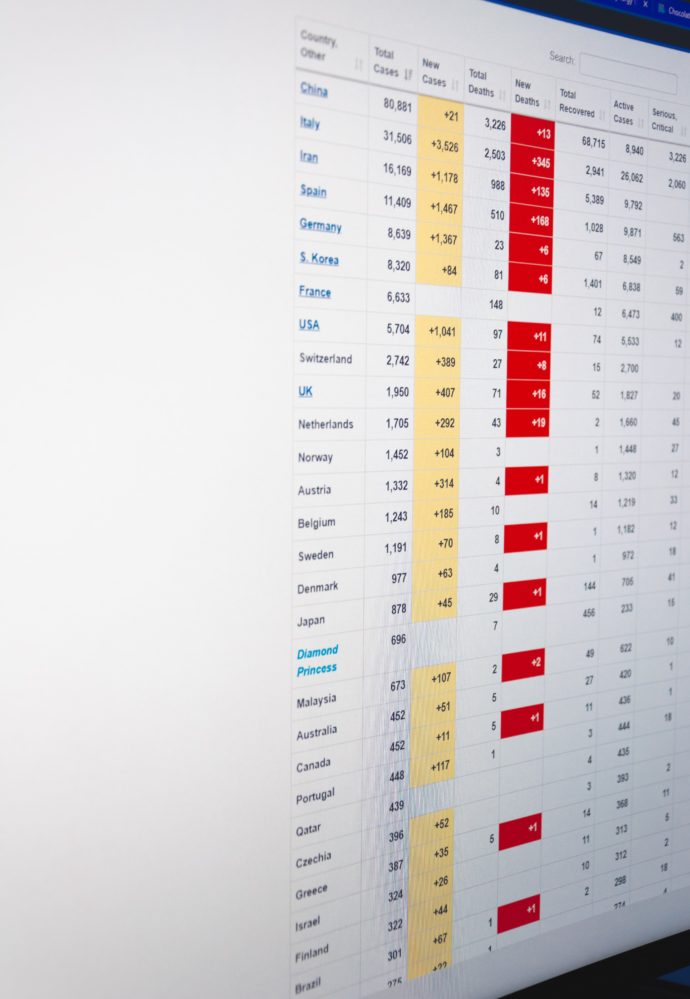
Midweek update
Today, the FFCRA Paid COVID-19 Sick Leave Benefit took effect generally for private employers with under 500 employees. The Labor Department which enforces this law published temporary rules on mandated benefit. The informal guidance accompanying the rules explains that
Most employees of the federal government are covered by Title II of the Family and Medical Leave Act, which was not amended by this Act, and are therefore not covered by the expanded family and medical leave provisions of the FFCRA. However, federal employees covered by Title II of the Family and Medical Leave Act are covered by the paid sick leave provision.
Federal News Network discusses the complex impact of COVID-19 on the U.S. Postal Service. The article begins as follows:
With the Postal Service now tracking the deaths of its employees from the coronavirus pandemic, in addition to a growing list of those who have tested positive for the virus, the agency is doing everything it can to continue normal operations — even in the most extraordinary circumstances.
The USPS Board of Governors held a moment of silence Wednesday for postal employees who have died of complications from COVID-19, the illness caused by the current strain of the virus.
Those include Rakkhon Kim, a 50-year old letter carrier in New York City, who died of complications from the virus last week.
Terribly sad.
It’s worth linking to this Wall Street Journal article on nurses working on the COVID-19 front line in the Bronx, New York. The Boston Globe’s STAT discusses how the COVID-19 spreads and creates additional hot spots.
Healthcare Dive reports on a FAIR Health study of consumer use of telehealth and retail clinics relying on data gathered before the current COVID-19 emergency. “Consumer use of telehealth and retail clinics spiked from 2017 to 2018, while use of urgent care centers, ambulatory surgery centers and emergency rooms dropped as consumers increasingly turn to cheaper sites of care for low-acuity medical needs.”
As the article notes, telehealth use has become a necessity in the past month. HIMSS provides a patient guide on how to get the most out of telehealth visits. This may be useful for health plans to share with members.