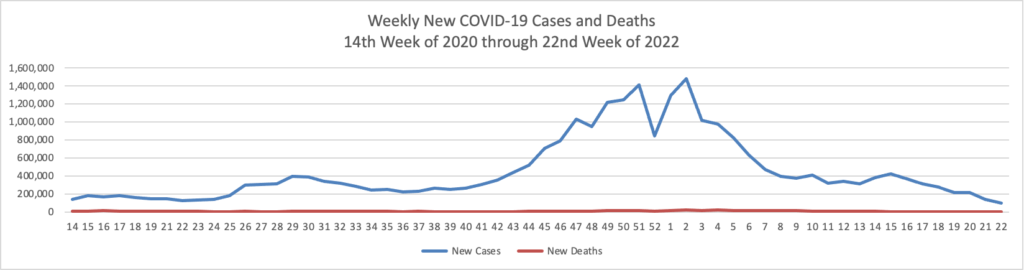
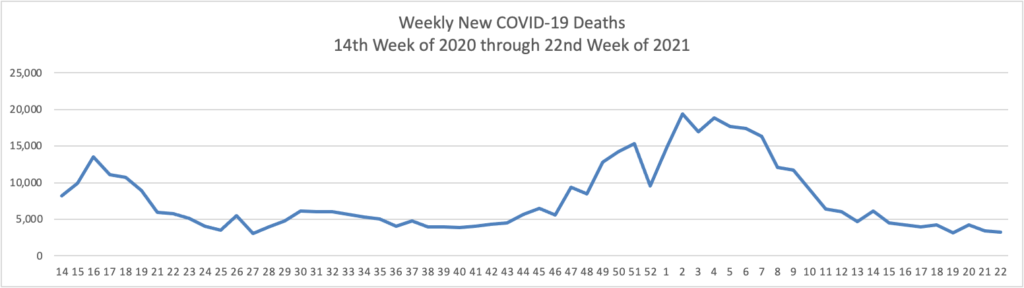
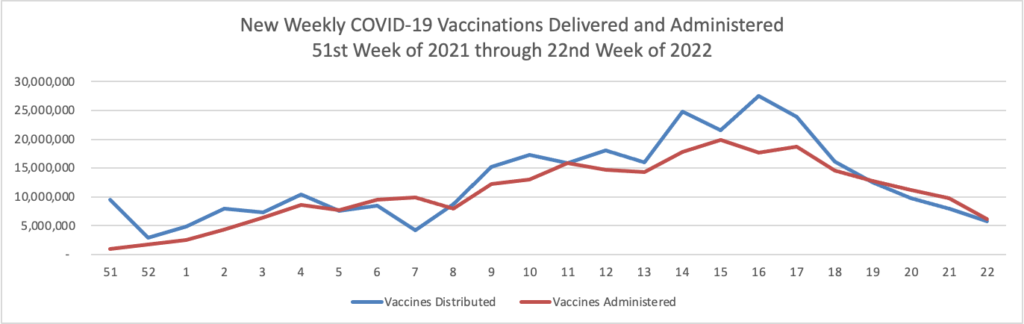
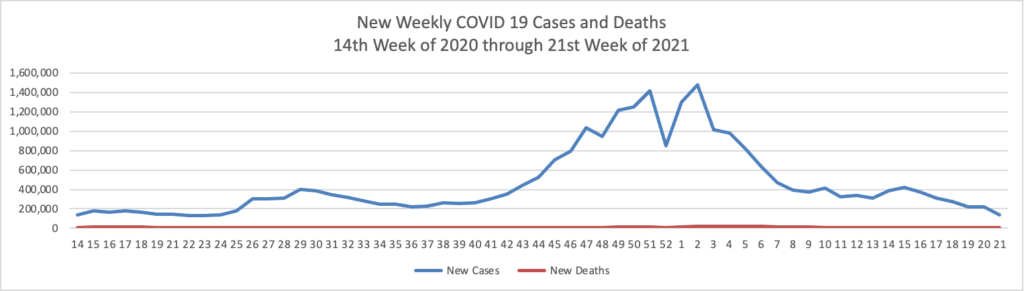
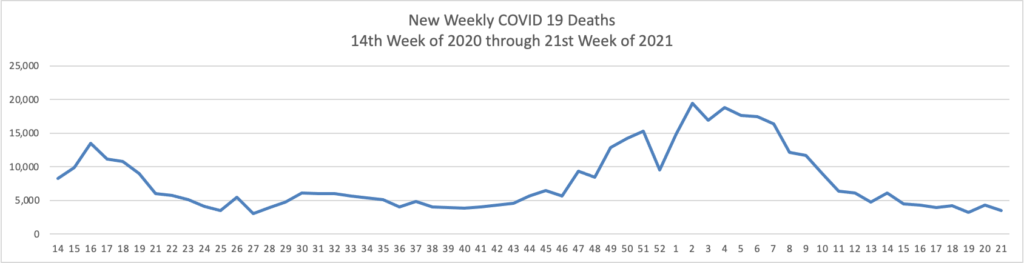
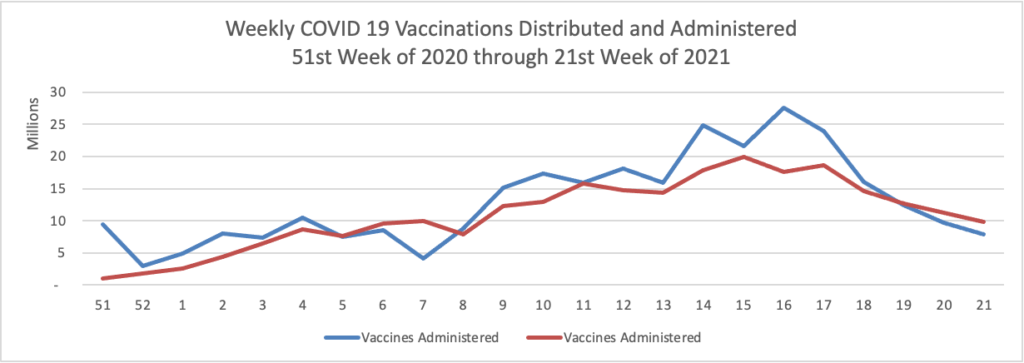
Tuesday’s Tidbits

The Blue Cross Blue Shield Association announced today that
the Blue Cross and Blue Shield (BCBS) Government-wide Service Benefit Plan, also known as the Federal Employee Program® (FEP®) announced it will launch a COVID-19 Vaccination Incentive Program starting Friday, June 11, 2021, to encourage members to get vaccinated against COVID-19 and help meet President Biden’s goal of having at least 70% of the U.S. adult population receive one or more vaccinations for COVID-19 by July 4, 2021.
Eligible members – those over the age of 18 with an FEP MyBlue® account – will receive $50 on their MyBlue Wellness Card when they provide official documentation of their COVID-19 vaccination record. Members can use these incentive funds to purchase qualified medical expenses. FEP is the first Federal Employees Health Benefit program to offer a vaccination incentive like this to its members.
In other COVID-19 news —
- The Wall Street Journal reports that
Pfizer and partner BioNTech SE said Tuesday that they have begun testing their vaccine in children under 12 years old in a pivotal study. If the results prove positive, Pfizer said it would ask U.S. health regulators in September to expand use to some of the younger children.
Meantime, Moderna Chief Executive Stephane Bancel said results of testing Moderna’s vaccine in children as young as five years could become available by the fall, which if positive could lead to regulatory authorization of its use in the younger age group.
- Dr. Marty Makary writes in a Journal op-ed that “The news about the U.S. Covid pandemic is even better than you’ve heard. Some 80% to 85% of American adults are immune to the virus: More than 64% have received at least one vaccine dose and, of those who haven’t, roughly half have natural immunity from prior infection. There’s ample scientific evidence that natural immunity is effective and durable, and public-health leaders should pay it heed.”
- The Journal also reports that
Hospitals, state health departments and the federal government are racing to decide how to use up millions of Johnson & Johnson’s vaccine doses that are set to expire this month.
The prospect of so many doses going to waste in the U.S. when developing nations are desperate for shots would add pressure on the Biden administration to share stockpiled vaccines. But there are few practical solutions to administering them quickly in the U.S. or distributing them in time to foreign countries, according to those involved in the vaccination drive.
The stockpile is, in part, an unintended consequence of the U.S.’s decision in April to temporarily suspend administration of J&J doses to assess a rare blood-clot risk. The pause forced states and providers to cancel large blocks of appointmentsthat were never rescheduled, leaving a surplus of supply, and in some areas increasing hesitancy over the J&J vaccine’s safety, according to industry officials. * * *
The issue of expiring doses is the latest setback for J&J’s Covid-19 vaccine effort. An accident at a contract manufacturer’s plant led to the contamination of material that could have yielded up to 15 million doses and led to a halt in production of the J&J vaccine there.
This serious Johnson & Johnson one dose COVID-19 vaccine distribution problem impairs efforts to vaccinate people like the unhoused who are unlikely to return for a second dose of the mRNA vaccine.
Following up on yesterday’s news about Food and Drug Administration of an Alzheimer’s Disease drug, the Wall Street Journal offers a medical specialist’s observations on this terrible disease. Also Fierce Healthcare informs us that Biogen has “unveiled collaborations with CVS Health, Cigna to boost access to its Alzheimer’s drug.”
The Washington Post informs us that
Senate Republicans are blocking a quick confirmation for President Biden’s nominee to lead the federal personnel agency, targeting her past emphasis on the concept of systemic racism known as “critical race theory” that has become a lightning rod for conservatives.
Republicans also are pushing back on Kiran Ahuja’s support for abortion rights at a time when a long-standing ban on federal funding for the procedure — known as the Hyde Amendment — has emerged as a renewed flash point for the right because of Biden’s support for overturning it.
The delay on Ahuja’s nomination is being led by Sen. Josh Hawley (R-Mo.), although several Republicans objected to a quick confirmation vote for her, according to senior Democratic and GOP officials. The move will force Senate Majority Leader Charles E. Schumer (D-N.Y.) to go through procedural hurdles on the Senate floor, rather than move quickly with a pro forma vote that is more common for nominees to lower-profile posts.
In the tidbits department —
- Health Payer Intelligence reports
The majority of the average healthcare premium dollar goes toward prescription drugs and outpatient and inpatient hospital costs, according to AHIP’s most recent research.
AHIP looked at 30 commercial health insurance providers’ financial statements between 2016 and 2018 to get the breakdown of how payers were spending members’ premiums. The data included spending by employer-provided coverage and coverage that individuals can purchase on the healthcare market.
The biggest chunk of the healthcare dollar went towards prescription drugs, with 21.5 cents dedicated to payments for outpatient prescription medications and prescription medications administered in a physician’s office or clinic.
Prescription drugs are closely followed by spending on outpatient hospital and inpatient hospital services, both around 19 cents while medical services come in at 12 cents.
- Healthcare Dive informs us that yesterday “Apple unveiled new health features aimed at patient-doctor data exchange.” Moreover, “Apple is launching new mobility capabilities for its iPhone. Using information from its motion sensors, the phone can now collect data on someone’s balance, stability and coordination by measuring stride length and timing, and warn them they might be at risk for a harmful fall if they’re walking unsteadily. Usually, fall risk is assessed by a provider using a questionnaire and in-person exam.” The new features will be available on Apple devices in the fall of this year.
- According to a Department of Health and Human Services press release, “The White House, the U.S. Department of Health and Human Services (HHS) Office of the Assistant Secretary for Preparedness and Response (ASPR) and the U.S. Food and Drug Administration (FDA) today released a series of policy recommendations to address the vulnerabilities in U.S. pharmaceutical supply chains. Led by FDA and ASPR, the White House report – PDF and its recommendations have been accepted by President Biden.”
“To secure the supply chain, the report’s recommendations center on four pillars:
- Boosting local production and fostering international cooperation;
- Promoting research and development that establishes innovative manufacturing processes and production technologies to strengthen supply chain resilience;
- Creating robust quality management maturity to ensure consistent and reliable drug manufacturing and quality performance, and
- Leveraging data to improve supply chain resilience.”