Welcome Director Ahuja

OPM’s new Director Kiran Ahuja was sworn in today. Here is a link to the OPM press release on the festivities.
Health Payer Intelligence informs us that “The Alliance of Community Health Plans (ACHP) has proposed a number of recommendations to improve the Federal Employees Health Benefits (FEHB) program’s plan comparison tool in order to boost quality and enrollment, according to a recent issue brief.” ACHP’s action is timely because OPM has been focusing attention on the plan comparison tool in consultation with interested carriers and presumably other stakeholders.
According to a Committee press release, “The House Appropriations Subcommittee on Financial Services and General Government today approved by voice vote its fiscal year 2022 bill. [This is the bill that funds OPM and the FEHB.] For fiscal year 2022, the draft bill includes $29.1 billion in funding, an increase of $4.8 billion over 2021.”
Sen. Chuck Grassley (R Iowa) announced
Sen. Chuck Grassley (R-Iowa) today joined Senate Majority Whip Dick Durbin (D-Ill.) Sen. Angus King (I-Maine) to introduce the Drug-price Transparency for Competition (DTC) Act, a bill that would require price disclosures on advertisements for prescription drugs, in order to empower patients and reduce spending on medications. Last week, the Government Accountability Office (GAO) released a report – requested by Durbin and Grassley – which found direct-to-consumer (DTC) advertisements of prescription drugs contribute to an enormous amount of Medicare costs. Specifically, the DTC Act would require DTC advertisements for prescription drugs and biological products to include a disclosure of the list price, so that patients can make informed choices when inundated with drug commercials.
Speaking of drug prices, let’s take a look at recent news on the new Alzheimer’s Disease drug, Aduhelm.
- Yesterday, Biogen issued a bulleted defense of its pricing, which is $56,000 annually per patient. STAT News points out “For families and physicians grappling with the historic approval this month of the controversial Alzheimer’s drug Aduhelm, there’s no shortage of unanswered questions. But a critical one has largely been overlooked: Once patients start taking the medication, how will they know when it’s time to stop? “We don’t have any guidance on how long to give this medication to someone who doesn’t experience adverse events,” said William Mantyh, a behavioral neurologist at M Health Fairview University of Minnesota Medical Center. “With a drug like aducanumab where the upfront demonstrated efficacy is up in the air, it really makes it hard for a clinician to figure out when to stop the drug based on a patient’s clinical symptoms.”
- Axios interviewed AHIP CEO Matt Eyles on Aduhelm pricing. In response to an Axios question on acceptable pricing, Mr. Eyles responded that “The best information we have is what [the Institute for Clinical and Economic Review] puts out.” ICER stated on June 7 that “At the ICER public meeting on aducanumab on July 15, 2021, we will tackle important questions [about Aduhem] with all stakeholders at the table. We will also address the question of fair pricing for a drug that now seems likely to become one of the top selling drugs in the history of the United States. ICER’s preliminary draft report calculated a fair annual price to lie between $2,500-$8,300. Even in our most optimistic cost-effectiveness scenario — which ignores the contradictions within the two pivotal trials and presumes that only the positive trial captures the true benefits of treatment — aducanumab’s health gains would support an annual price between $11,100-$23,100. The list price of $56,000 per year announced today by the drug maker far exceeds even this optimistic scenario. Our report notes that only a hypothetical drug that halts dementia entirely would merit this pricing level. The evidence on aducanumab suggests that, at best, the drug is not nearly this effective. Nonetheless, even at the lower range of the estimated number of eligible patients, at this price the drug maker would stand to receive well in excess of $50 billion per year even while waiting for evidence to confirm that patients receive actual benefits from treatment.
- The Wall Street Journal reports that “Eli Lilly & Co. plans to submit its Alzheimer’s drug for market clearance under an expedited review this year, in a sign that regulators are encouraging development of treatments for the disease after a recent approval. Lilly said Thursday that the U.S. Food and Drug Administration had designated the company’s experimental Alzheimer’s drug, called donanemab, for the agency’s accelerated approval process. The FDA decision comes after the agency cleared Biogen Inc.’s Aduhelm, the first Alzheimer’s therapy to receive approval in nearly two decades but one that has drawn criticism from doctors and researchers skeptical the drug works. * * * Donanemab performed better in a trial than Biogen’s drug did in its trials, and health insurers and patients would probably prefer it over Aduhelm, J.P. Morgan analyst Chris Schott said in a note to investors.“Donanemab’s approval would be a major blow to Aduhelm’s commercial prospects,” Brian Skorney, a Robert W. Baird & Co. analyst, said in a research note. “We think it would make zero sense for FDA to approve Aduhelm, but not donanemab.” Ah, competition.
In other drug pricing news, Fierce Healthcare tells us that
Cigna is launching a new program that aims to incentivize eligible members to switch to biosimilar drugs.
Under the new Shared Savings Program, members will be offered a one-time $500 debit card for healthcare services or medications if they make the decision to switch to a biosimilar, according to an announcement provided first to Fierce Healthcare.
The program will be made available first to [approximately 7,000] eligible patients taking Remicade, a brand-name biologic that treats a number of inflammatory conditions such as Crohn’s disease and psoriasis. Remicade infusion costs can vary, but Cigna claims data suggest the average regimen costs $30,000 per year, with expenses growing depending on the site of administration.
Two biosimilars for the drug, Avsola and Inflectra, will be moved to the insurer’s preferred tier in July. Eligible customers and their providers will be notified by Cigna about their eligibility to participate in the Shared Savings Program in the coming weeks, the insurer said.
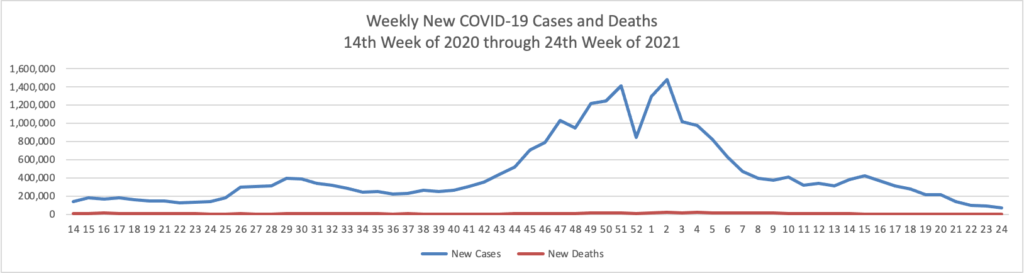
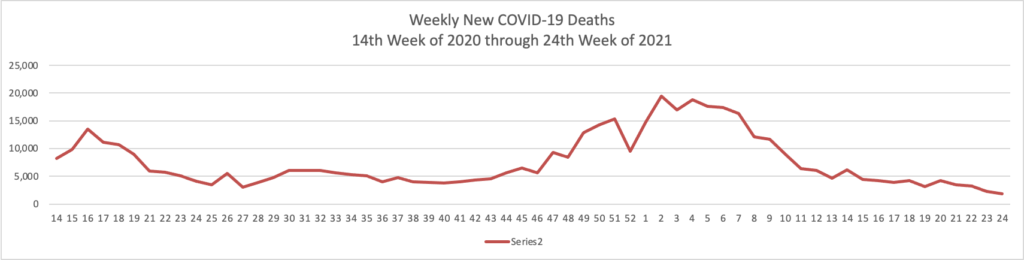
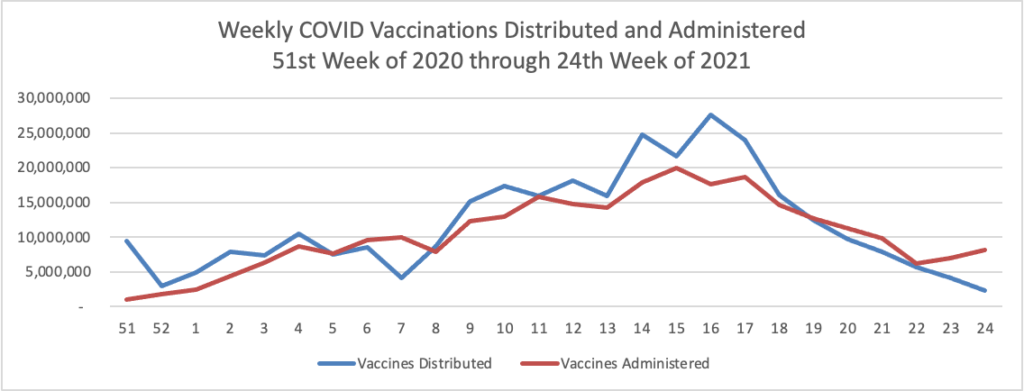
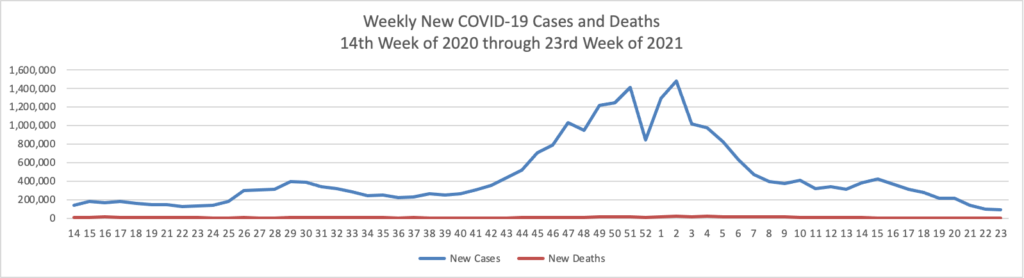
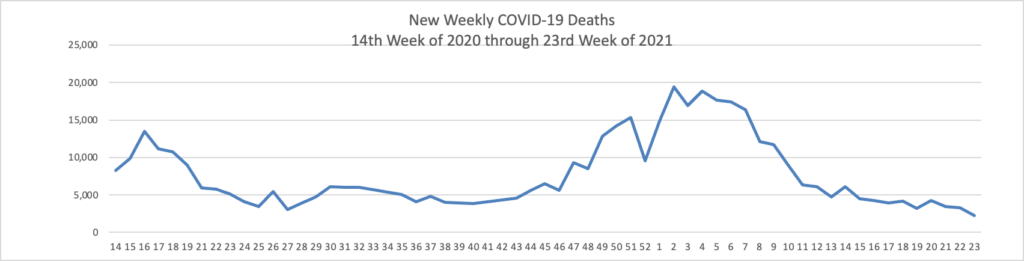
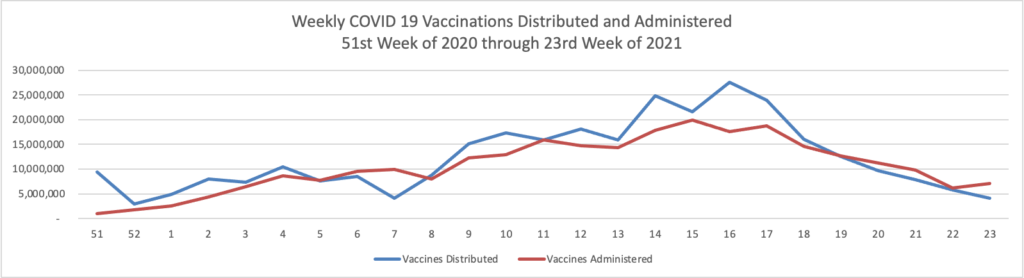
In COVID-19 news —
- Fierce Biotech reports that “The FDA green-lit its first antibody test that doesn’t use blood samples to check for evidence of a COVID-19 infection and instead relies on simple, painless mouth swabs. Developed by Diabetomics, the rapid, lateral-flow diagnostic received an agency emergency authorization allowing it to be used at the point of care for adults and children. Designed to deliver a result within 15 minutes, the CovAb test also does not require any additional hardware or instruments. When administered at least 15 days after the onset of symptoms, when the body’s antibody response reaches higher levels, the test demonstrated a false-negative rate of less than 3% and a false-positive rate of nearly 1%, according to the company.”
- The New York Times reports that the Baltimore Maryland factory that had been producing the single dose Johnson & Johnson COVID-19 vaccine remains shuttered which Congress investigates its owner Emergent Biosolutions.
- The NIH Director’s blog informs us about new NIH research on how Immunity generated from COVID-19 vaccines differs from an Infection. “The good news so far is that, unlike the situation for the common cold, we have now developed multiple COVID-19 vaccines. The evidence continues to suggest that acquired immunity from vaccines still offers substantial protection against the new variants now circulating around the globe. The hope is that acquired immunity from the vaccines will indeed produce long-lasting protection against SARS-CoV-2 and bring an end to the pandemic. These new findings point encouragingly in that direction. They also serve as an important reminder to roll up your sleeve for the vaccine if you haven’t already done so, whether or not you’ve had COVID-19. Our best hope of winning this contest with the virus is to get as many people immunized now as possible. That will save lives, and reduce the likelihood of even more variants appearing that might evade protection from the current vaccines.” Amen to that.
In a bit of Thursday miscellany
- Patient Engagement reports that “Optum is bringing healthcare right into Utah’s backyard, rolling out a new Optum Mobile Health Clinic to improve care access for individuals in Optum Care Network Utah. The mobile health clinic, a 45-foot-long vehicle with two private exam rooms, a waiting room, and an imaging lab, is set to address the leading care access barriers experienced by Utahns.” Well done.
- A friend of the FEHBlog called his attention to the NIH report on an engaging study suggesting scientists may need to rethink which genes control aging.