Weekend update

The Senate will resume Committee work and floor voting this coming week while the House of Representatives will be limited to Committee business.
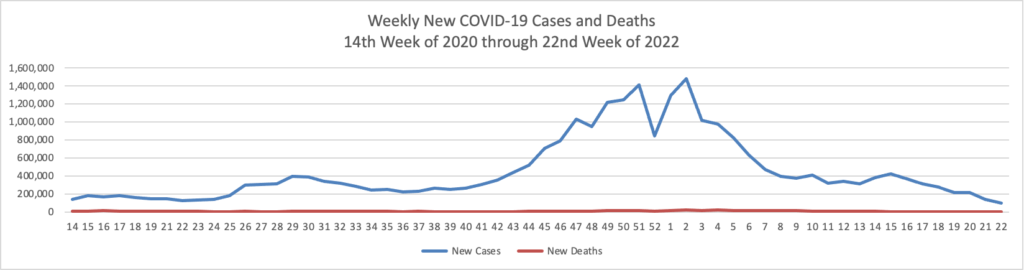
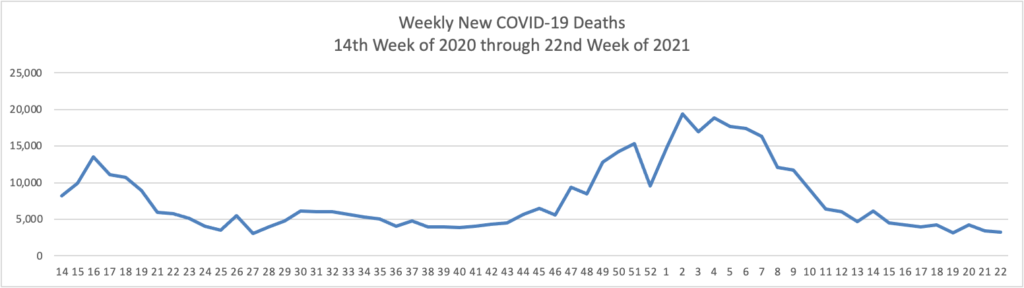
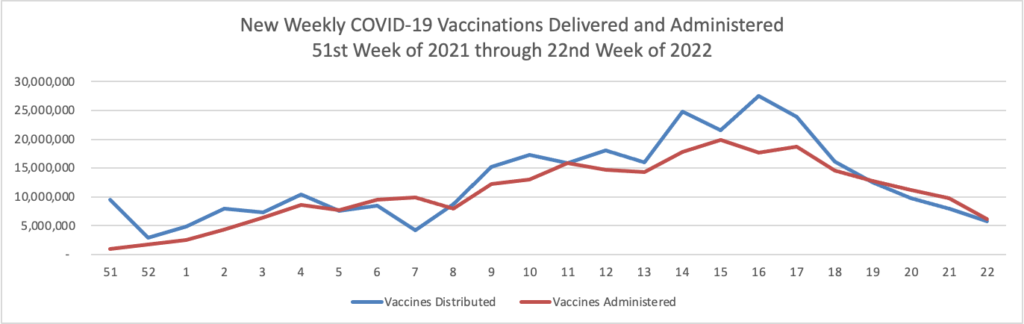
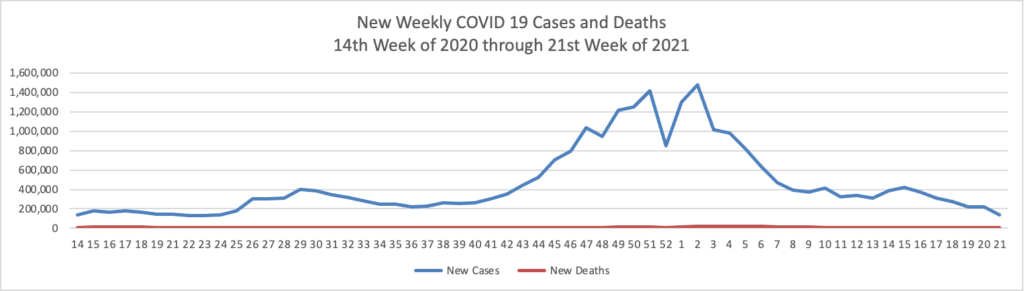
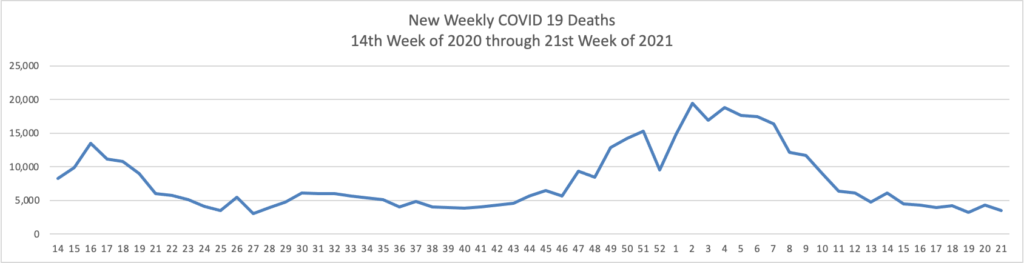
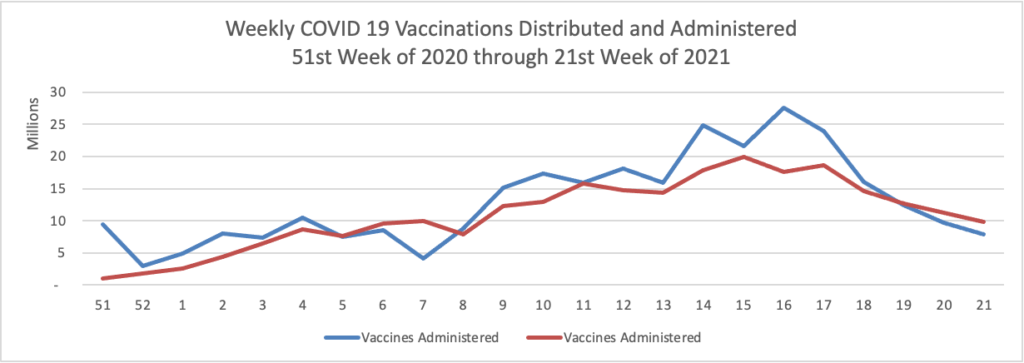
From the COVID-19 front
- Bloomberg reports that “U.S. hospitalizations continue to fall, with 3.17% of beds occupied by Covid-19 patients on June 4, according to the U.S. Department of Health and Human Services. That percentage dropped from 3.67% on May 28 and is the lowest since March 14, 2020.”
- The Advisory Board informs us its June 4, 2021, COVID-19 Roundup that “The World Health Organization (WHO) on Monday announced variants of the coronavirus will now be named after letters of the Greek alphabet, to simplify the variants’ names and avoid names that can be stigmatizing to a country. According to the new naming system, the variant B.1.1.7, which was first discovered in the United Kingdom, will now be called Alpha; the B.1.351 variant first discovered in South Africa will be called Beta; and the B.1.617.2 variant first discovered in India will be called Delta. Once all 24 letters of the Greek alphabet have been used, WHO said it will announce another naming system.”
Kaiser Health News tells us that
The Food and Drug Administration’s decision next week whether to approve the first treatment for Alzheimer’s disease highlights a deep division over the drug’s benefits as well as criticism about the integrity of the FDA approval process.
The agency said it will decide by June 7 the fate of Biogen’s drug aducanumab, despite a near-unanimous rejection of the product by an FDA advisory committee of outside experts in November. Doubts were raised when, in 2019, Biogen halted two large clinical trials of the drug after determining it wouldn’t reach its targets for efficacy. But the drugmaker later revised that assessment, stating that one trial showed the drug reduced the decline in patients’ cognitive and functional ability by 22%.
Some FDA scientists in November joined with the company to present a document praising the intravenous drug. But other FDA officials and many outside experts say the evidence for the drug is shaky at best and that another large clinical trial is needed. A consumer advocacy group has called for a federal investigation into the FDA’s handling of the approval process for the product.
A lot is riding on the drug for Biogen. It is projected to carry a $50,000-a-year price tag and would be worth billions of dollars in revenue to the Cambridge, Massachusetts, company.
The Department of Health and Human Services, last Friday, released
a new report that shows 31 million Americans have health coverage through the Affordable Care Act – a record. The report also shows that there have been reductions in uninsurance rates in every state in the country since the law’s coverage expansions took effect. People served by the health Marketplaces and Medicaid expansion have reached record highs.
The data shows those individuals currently enrolled in health coverage through the Health Insurance Marketplaces and Medicaid expansion under the ACA, including 11.3 million people enrolled in the ACA Marketplace plans as of February 2021 and 14.8 million newly-eligible people enrolled in Medicaid through the ACA’s expansion of eligibility to adults as of December 2020.
In addition, there are one million people enrolled in the ACA’s Basic Health Program, and nearly four million previously-eligible adult Medicaid enrollees who gained coverage under expansion due to the ACA’s enhanced outreach, streamlined applications, and increased federal funding under the ACA. Today’s report shows the important role the ACA has played in providing coverage to millions of Americans nationwide.
The report also shows that between 2010 and 2016, the number of nonelderly uninsured adults decreased by 41 percent, falling from 48.2 million to 28.2 million. All 50 states and the District of Columbia have experienced reductions in their uninsured rates since the implementation of the ACA, with states that expanded Medicaid experiencing the largest reduction in their uninsured rate. California, Kentucky, New York, Oregon, Rhode Island, Washington, and West Virginia have reduced their uninsured rate by at least half from 2013 to 2019 through enrollment in Marketplace coverage and expansion of Medicaid to adult populations. To date, 37 states and the District of Columbia have expanded Medicaid to cover adults under the ACA.
Healthcare Dive reports that
- The nation’s largest commercial insurer is taking a closer look at whether visits to the emergency room by some of its members are necessary. Starting July 1, UnitedHealthcare will evaluate ER claims using a number of factors to determine if the visit was truly an emergency for its fully insured commercial members across many states, according to a provider bulletin.
- If UnitedHealthcare finds the visit was a non-emergency, the visit will be “subject to no coverage or limited coverage,” the provider alert states.
- However, a statement provided to Healthcare Dive said the insurer will reimburse for non-emergency care according to the member’s benefit plan. In other words, the amount paid by UnitedHealthcare may be less if deemed a non-emergency.
For what it’s worth, this plan designed to control healthcare resources makes sense to the FEHBlog.
Last week, the FEHBlog noted that OPM had a settled a lawsuit in the National Federation for the Blind alleged that the agency’s website was not adequately accessible to visually impaired FEHB members. To place the settlement in context, the FEHB calls attention to this NextGov article reporting that
Federal websites are not as accessible for those with disabilities as the law mandates they should be, according to a report released Thursday by the Information Technology and Innovation Foundation.
The report tested the 72 most popular federal websites and used a combination of automated tests and qualitative assessments to assess their compliance with Section 508 of the Rehabilitation Act. The law requires the General Services Administration to ensure federal websites are accessible to people with disabilities, including federal employees and the public.
According to the report, 30% of the most popular federal websites did not follow modern web accessibility standards on their homepages, and 48% failed a standard test on at least one of their three most popular web pages.
The report finds that
Overall, our assessments reveal a large amount of variation in how agencies are meeting Section 508 requirements. Some agencies that have a large footprint—such as the Internal Revenue Service, the Census Bureau, the Department of Defense, and the Department of Education’s office of Federal Student Aid—scored low in our accessibility test of their websites, indicating that people with disabilities may have difficulty accessing essential government services or information about these services online. * * *
Notably, the White House and the Centers for Disease Control and Prevention earned a perfect score in our accessibility test of all three of their pages, and also performed well in our qualitative assessment. The Biden administration has committed to adhering to WCAG 2.1 Level AA criteria on the White House website—a step above Section 508’s requirements, which use WCAG 2.0.19