Because it’s the beginning of the three day weekend, the Centers for Disease Control did not publish its weekly review of CDC Covid statistics. The FEHBlog values that weekly review because it complements the FEHBlog’s own Covid charts which are based on the CDC’s Covid Data Tracker and use Thursday as the first day of the week. So without further ado, here are the FEHBlog’s three Covid charts for this week, the 35th week of 2022:
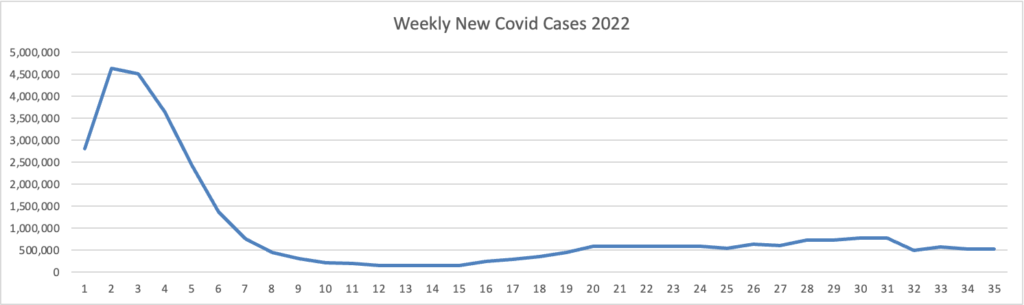
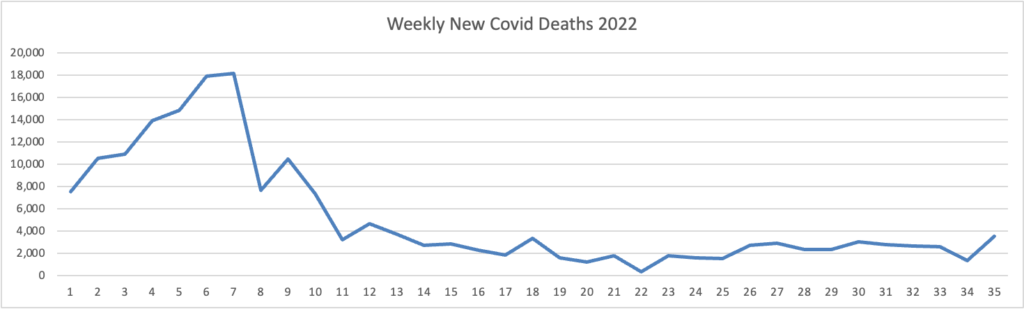
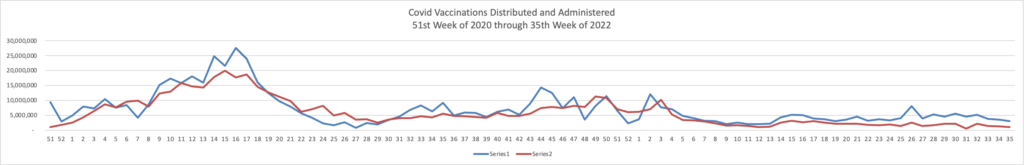
It’s always worth noting that the peaks on the left sides of the top two charts reflect the grand daddy of surges, the first Omicron wave. With respect to Covid vaccines, Revcycle Intelligence reports that the American Medical Association has added eight new CPT-4 codes to its procedures manual to cover physician services for the new Covid bivalent boosters.
From the U.S. healthcare business front, the Wall Street Journal reports this evening
CVS Health Corp. is in advanced talks to acquire the home-healthcare company Signify Health Inc. for around $8 billion, according to people familiar with the matter.
CVS appears to have beat out other heavy hitters including Amazon.com Inc. and UnitedHealth Group Inc., which had been circling Signify for a deal that could be announced soon. UnitedHealth never submitted an official bid, one of the people said.
There is still no guarantee that CVS will reach a deal for Signify, which has been exploring strategic alternatives since earlier this summer.
While Signify’s deadline for bids is Tuesday, September 6, Signify could strike a deal before then.
UHC is not sitting on its hands though. According to MedPage Today
UnitedHealth Group’s healthcare services division, Optum, will reportedly acquire Kelsey-Seybold for approximately $2 billion, according to the Star Tribune.
The acquisition of Kelsey-Seybold, a physician group based in Houston that includes cancer and women’s health centers, two ambulatory surgery centers, and a sleep center, was first announced in April, but few details have emerged since, the Star Tribunereported.
However, Optum, based in Eden Prairie, Minnesota, did provide a statement to MedPage Today about incorporating Kelsey-Seybold’s operations and staff into the Optum network.
From the rather quiet monkeypox front, STAT News raises a new concern.
A new study is raising concerns about the effectiveness of the monkeypox vaccine being used in the United States and other parts of the world.
The work, which has not yet been peer-reviewed, found that two doses of the vaccine induced relatively low levels of neutralizing antibodies against the monkeypox virus, and those antibodies had poor neutralizing capacity.
The researchers noted the so-called correlates of protection — what is needed, in terms of immune system weaponry, to be protected against monkeypox — are not known. Still, the evidence of low levels of neutralizing antibodies raises questions about how much protection is generated by two doses of the vaccine, marketed as Jynneos in the U.S. and made by the Danish manufacturer Bavarian Nordic. * * *
The study also casts a shadow over the recent decision by the U.S. government and others to stretch vaccine supplies by giving people one-fifth of a regular dose — and to do so by intradermal (into the skin) rather than subcutaneous (under the skin) injection. Intradermal administration, which requires smaller doses to be protective, has been shown to be effective in other disease outbreaks with other types of vaccine.
From the opioid epidemic front, STAT News features an interview with Rahul Gupta, M.D., “the director of the White House Office of National Drug Control Policy and the first doctor to hold that position.”
Gupta’s selection as director of the White House Office of National Drug Control Policy, however, has ushered in a new era of federal drug policy. As the first doctor to hold the position, he says he will embrace new strategies, including harm reduction tactics, which aim to reduce drug users’ risk of overdose, death, and disease in lieu of a hardline, abstinence-only attitude.
Still, though, addiction treatment is dogged by stigma, underuse of existing medications, and ongoing debate about certain harm-reduction techniques. The debate came to a head last week in California, where Gov. Gavin Newsom vetoed a bill to allow supervised injection sites — essentially clinics where people can use illicit drugs under medical supervision so as to prevent overdose.
Gupta sat down with STAT this week to discuss the ongoing crisis and the Biden administration’s efforts to address it. While circumspect about Newsom’s decision, Gupta did take several positions that are far more aggressive than any of his predecessors: Calling out doctors for their role in poor treatment outcomes; arguing that the addiction medication buprenorphine is widely misunderstood; and advocating for contingency management, a new addiction intervention that offers rewards — often cash — in exchange for cessation of drug use.
From the public health front, the Food and Drug Administrations discusses “Using A Whole-Of-Governments Approach to Combating Illicit Health Products.” Among other steps, “the agency has partnered with the Organisation of Economic Co-Operation and Development (OECD) Task Force on Countering Illicit Trade, which has been studying the problem of illicit trade for 15 years.”
From the Rx coverage front, Endpoints News informs us
Last July, the cost-effectiveness drug watchdog ICER released a preliminary draft report that Vivus’ Qsymia was the most cost-effective option for weight loss. That decision has now been validated.
ICER made its final determination Wednesday [August 31] that Qsymia, the brand name for the appetite suppressant phentermine combined with anticonvulsant topiramate, was more cost-effective for weight loss than other competitors such as Novo Nordisk’s Saxenda (liraglutide) and Wegovy (semaglutide) or Currax Pharmaceuticals’ Contrave (bupropion/naltrexone). * * *
ICER reviewers also added that if Qsymia wasn’t performing well in certain patients, Contrave may be the next best cost-effective option. The reviewers noted in their report that there were a few limitations to analysis, including the full impact of the drugs in patients with chronic kidney diseases or conditions where weight loss may be beneficial.
